Sample planning
- Sample Mounting
- Mount and can material considerations
- Powder Sample Cans
- Single Crystal Mounts
- Sample Thickness
- Absorbing samples
- Standard Samples
Sample Mounting
There are two standard attachment methods for sample environment used on ARCS. Most sample environments provide 5/16-18 threads. The other choice is a 1/4” post.
Mount and can material considerations
The materials that go into a mount depend on several factors, the total scattering of the mount material, the desired temperature range of the experiment, and the materials touching the mount.
Most mounts are made of Al 6061 as, compared to Al 1100 (pure Al), it is almost as transparent, much stronger, has a higher annealing temperature, and the super conducting transition is suppressed below 300 mK. Therefore it works well for 300 mK < T < 300 K. Though Al is relatively transparent, scattering from it will still be observed. So the thickness of Al in the beam should be minimized as much as possible. More details on the scattering from Al are provided below in the Al description.
Any materials containing H are to be avoided as H as a large incoherent cross section. For spectroscopy V is generally avoided as well because of the large incoherent cross section.
Materials for measurements at temperatures below 300 K
In the temperature range between 10K and 300K, mounts are generally made of Al 6061. A vented can or an Al foil pouch are usually used as a secondary heat shield.
Below 10K more care needs to be taken to ensure good thermal contact. In these cases He exchange gas is used. For the bottom loading sample environments, (CCRs, He3 inserts, Dil fridges) a can is sealed in a glove box with 1 Atm of He for exchange gas. For the top loaders (CCRs, orange cryostats) the He can be controlled to a specific partial pressure, usually around 50 mTorr.
If operating at temperatures in the 10s of mK, where Al may go superconducting, OFHC Cu should be used instead for cans and mounts.
Materials for measurements at temperatures above 300K
When measurements are planned for temperatures above 300K, a more careful examination of the materials used in the experiment must be undertaken. A high temperature checklist and offline tests will be required to ensure that the procedure will work. Here is a summary of what needs to be considered.
-
Melting point of all materials in the sample space
-
The vapor pressure of all materials in the sample space. (The Handbook Of Physics and Chemistry has tables for the vapor pressure of many materials used in mounting.)
-
Any eutectic that can be formed between materials that are in the sample space.
-
Does the mounting material anneal to a point of losing structural integrity?
Al often, but not always, works well with the hot stick for Temperatures below 670K (The annealing temperature for Al 6061) as long as a material that has a low temperature eutectic with Al is not in contact. For example Brass and Al do not mix well as the Zn in the Brass will react with the Al. Heat shields made of Al can be used above 670K because they are not holding anything that requires material strength. On the other hand, cans or sample mounts made of Al will lose their strength above the annealing temperature and therefore should not be used.
V, Mo and Nb are the materials of choice for mounts to go to higher temperatures. If the sample needs to be encapsulated, Quartz tubes often work well.
Powder Sample Cans
There are several standard powder cans. The most common used cans on ARCS are made of Al 6061. The following table summarizes the standard cans.
| Can label | Diameter | 1mm annulus | 2mm annulus | T range |
|---|---|---|---|---|
| 27 mm | 0.25 in | N | N | <300K |
| 27 mm | 0.375 in | N | N | <300K |
| 27 mm | 0.5 in | N | N | <300K |
| 27 mm | 0.625 in | Y | Y | <300K |
| Foil Seal | 1.25 in | Y | Y | <670K |
Note there two typical styles of Cans the “27mm” named for the 27mm diameter lid and the “Al foil seal” named for the type of seal used. Note that the 27mm diameter cans are Indium sealed and should not be used above room temperature. Pictures of the types of cans are shown in Figures 1 and 2, respectively
 |
 |
|---|---|
 |
|
| Figure 1. Pictures of can parts for the various 27mm cans |

Figure 2. Pictures of Can Parts for the Foil Seal Cans
The Al foil sealed cans are only for annular samples, some of the 27mm cans can use inserts for annular samples. For the smaller diameter 27mm cans, the benefit of making an annulus is negated by the additional Al. The inserts for the annular samples have a cone to help with filling as shown in Figures 1 and 2. The cone should be placed in the can before filling with the tip pointing up.
If your experiment will go above 300K careful consideration of the pressure in the can should be considered before deciding to seal the can.
Sealing cans
For a detailed Description of Sealing Cans go to: Sealing Powder Cans
Single Crystal Mounts
For mounting single crystals we usually recommend wiring a crystal to a thin Al plate and then clamping a plate per crystal to a standard mounting ring.
An example of a standard mount is given in Figure 5. In this example the plates are 0.015” (~0.4mm) thick
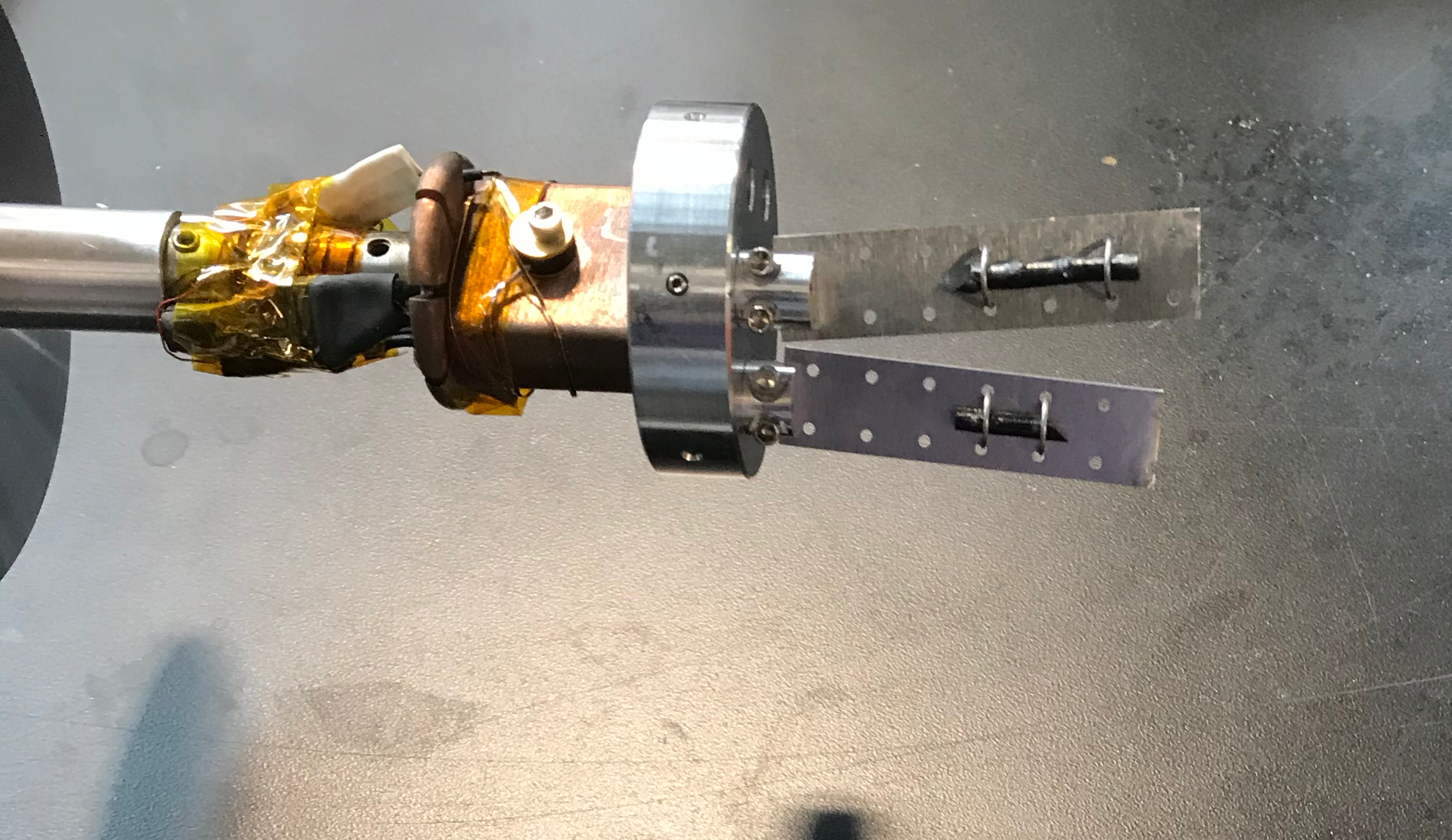 Figure 5 a view of a typical single crystal mount
Figure 5 a view of a typical single crystal mount
If an array of tiny crystals is being prepared, they are usually glued to an Al backplate. A fluorinated glue like Cytop is the preferred adhesive.
Single Crystals for Magnets
If the sample is a Ferromagnet, the design of the mount needs to be able to hold the force as the magnetic field is applied. Typical solutions are thicker Al and gluing the crystal or wiring it to constrain all motions.
Sample Thickness
To avoid multiple scattering the general rule is to design a sample as a 10% scatterer. To do this calculation one uses the total scattering cross section $\sigma_s$ which is available from the NIST website, a convenient way of accessing V. Sears Neutron News Article 1-e{-ρnσst} where ρn is the number density and t is the sample thickness.
However there are other factors to take into account when designing sample size. For example in a weak magnetic system you may try to maximize the scattering from the magnetic signal by making a thicker sample. This usually works because one only looks at low Q for magnetic signals and the multiple scattering is at high Q. However the user should be mindful of all scattering processes in their sample to identify spurious effects. For a counter example, look at Quantum harmonic oscillations in UN Here the excitations were observed at lower Q due to a multiple scattering between the excitation and incoherent process.
Absorbing samples
When designing an experiment the absorption cross section should be considered. Note if the absorption cross section is bigger than the scattering cross section, sometimes a smaller sample will result in more signal.
ARCS and SEQUOIA can operate in energy regions where resonances in the absorption spectrum can complicate measurements. Specific materials can be looked up at the IAEA website For example Ir has resonances around 700 and 900 meV. So an Ei of 500 meV for and Ir compound will work well and an Ei of 1000 meV will work well for energy transfers below 300 meV. However intermediate energies will not work so well.
Standard Samples
A set of standard samples is routinely measured on ARCS for instrument calibration or characterization.
V Cylinder
Measured every cycle for detector efficiency normalization and as instrument performance tests. This is a V cylinder that is 3cm tall 31.7mm in outer diameter and 27.9mm in inner diameter. (Or can be defined as a 1.25” pipe with a wall thickness is 0.150in) It is suspended by V wires and the mounting plate is shielded with B4C.

V Plate
Measured as needed for absolute units normalization.
Si Powder
Measured as needed for a diffraction standard.
Diamond powder
Measured as needed for a diffraction standard
Al
Measured to understand background considerations. Note the density of states of Al has a large peak near 20 meV and another peak around 38 meV.
ZrH2
Measured for the Neutron Scattering school and as an energy transfer standard.
C60
Measured as needed for long d spacing diffraction standard
C4H2I2S
Measured as needed. For finite energy transfer analysis.
